Explore our research
On this page, we list main projects that are currently run by members of the CSI lab.
TENSION Trial
Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window

TENSION was an investigator-initiated, randomized controlled, open label, blinded endpoint, European, two-arm, postmarket study to compare the safety and effectiveness of thrombectomy as compared to best medical care alone in stroke patients with extended stroke lesions. Patients were eligible, if they presented with an Alberta Stroke Program Early Computed Tomography Scan score of 3–5. The vision of TENSION was to reduce the burden of death and disability from stroke by providing innovative effective treatment to severe stroke patients for whom currently no effective treatment is available.
MAIN FINDINGS: From 2018 to 2023, 253 patients were randomly assigned, with 125 patients assigned to endovascular thrombectomy and 128 to medical treatment alone. The trial was stopped early for efficacy after the first pre-planned interim analysis. At 90 days, endovascular thrombectomy was associated with a shift in the distribution of scores on the modified Rankin Scale towards better outcome (adjusted common OR 2·58 [95% CI 1·60–4·15]; p=0·0001) and with lower mortality (hazard ratio 0·67 [95% CI 0·46–0·98]; p=0·038). Symptomatic intracranial haemorrhage occurred in seven (6%) patients with thrombectomy and in six (5%) with medical treatment alone.
Endovascular thrombectomy was associated with improved functional outcome and lower mortality in patients with acute ischaemic stroke from large vessel occlusion with established large infarct in a setting using non- contrast CT as the predominant imaging modality for patient selection.
TENSION receives funding by the European Union’s Horizon 2020 research and innovation programme.
Wake-up trial

WAKE-UP was a European multicentre investigator-initiated randomized placebo-controlled clinical trial of MRI based thrombolysis in acute stroke patients with unknown time of symptom onset, e.g. due to recognition of stroke symptoms on awakening.
WAKE-UP was a European collaborative research project launched by a consortium of academic and SME partners. The trial involved 70 study centers from 8 European countries. Of 1,362 patients screened by MRI, 503 were randomized to treatment with intravenous alteplase or placebo. The primary end point was favorable outcome, as defined by a score of 0 or 1 on the modified Rankin scale (mRS) of neurologic disability.
WAKE-UP demonstrated efficacy and safety of treatment with intravenous alteplase in patients with acute stroke with an unknown time of onset, who had a mismatch between diffusion-weighted imaging and FLAIR in the region of ischemia (DWI-FLAIR mismatch) (Thomalla et al., 2018). The results of the trial have led to changes in guideline recommendations and clinical practice. WAKE-UP received funding by the European Union Seventh Framework Program
ClinicalTrials.gov Identifier, NCT01525290
Wake-up MRI visual rating training tool
Hamburg City Health Study
The Clinical Stroke & Imaging Research (CSI) group leads the brain MRI analysis within the Hamburg City Health Study (HCHS), a large-scale epidemiological study aimed at understanding the complex interplay between genetics, environment, biology, and lifestyle in developing major diseases. HCHS seeks to identify risk factors for common conditions such as stroke, dementia, and cardiovascular diseases to enable earlier, more precise, and personalized treatments.
Our group leads the brain MRI analysis within the Hamburg City Health Study (HCHS), a large-scale epidemiological study aimed at understanding the complex interplay between genetics, environment, biology, and lifestyle in developing major diseases.
The study aims to identify risk factors for common conditions such as stroke, dementia, and cardiovascular diseases to enable earlier, more precise, and personalized treatments. Currently, cross-sectional data from 5000 participants with state-of-the-art brain MRI data and extensive imaging and clinical phenotyping of all major body systems is available. Longitudinal follow-up investigations are planned.
In the HCHS, we investigate high-risk individuals in early stages of Cerebral Small Vessel Disease to identify biomarkers for targeded preventive measures.
Brain Networks in Stroke Prognosis
Our group investigates disturbances of structural and functional brain networks within the collaborative research group 936 (SFB 936, Multi-Site Communication in the Brain). We curated the UKE stroke database, a longitudinal study of patients with first symptomatic stoke and upper extremity motor deficit, including a comprehensive clinical characterization and tailored state-of-the-art MRI examinations.
We examined the structural impact of a focal ischemic lesion within a network of interconnected sensorimotor brain areas using reconstruction of white matter tracts (DTI) and analysis of cortical thickness. Dynamics of structural alterations of grey and white matter within the sensorimotor network were related to clinical outcome. Findings of our study are integrated into a complex model of recovery from stroke with results from functional imaging and EEG analysis together with other projects in SFB 936.
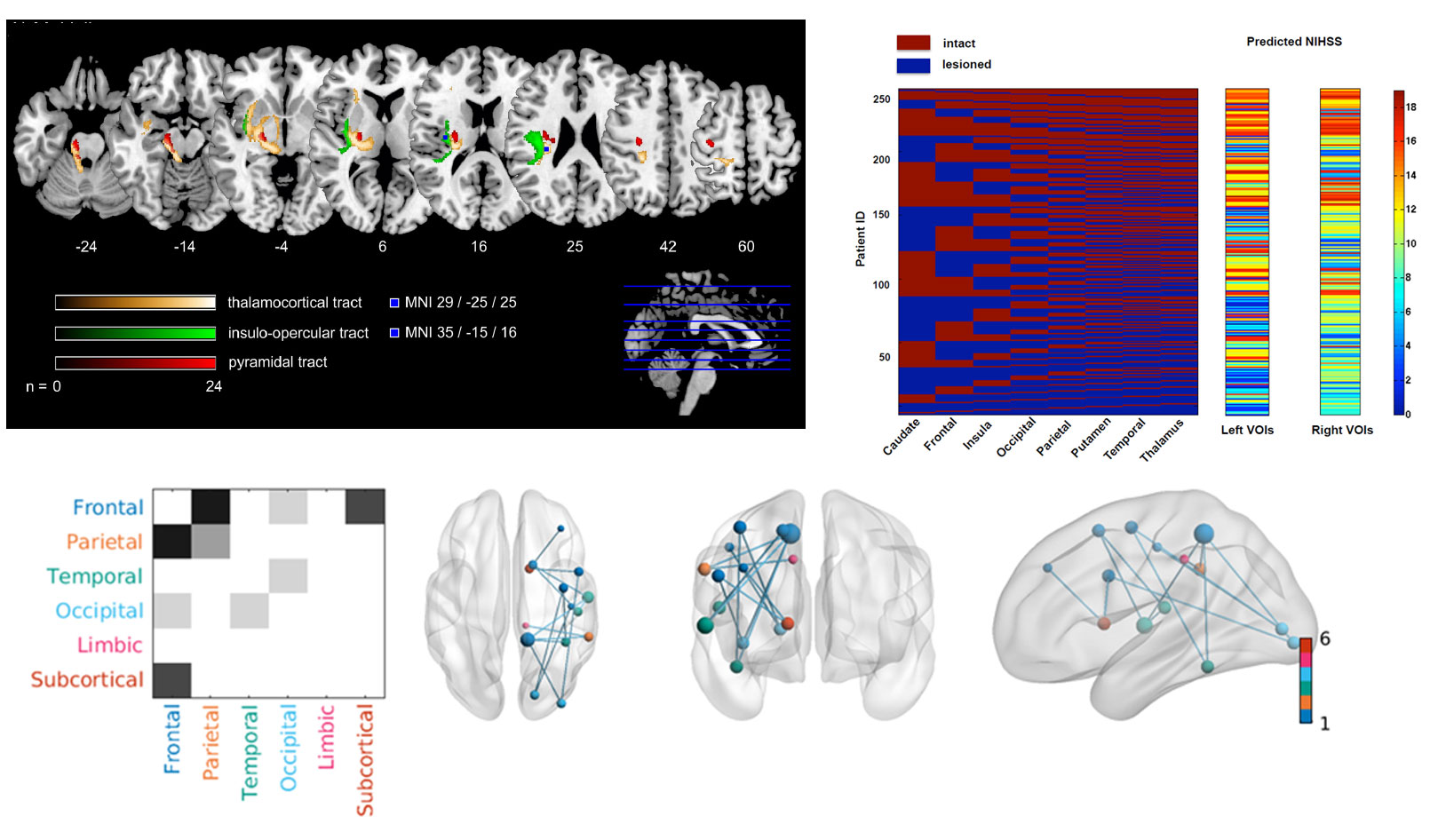
Multivariate lesion symptom mapping
We explore novel, innovative multivariate approaches, and lesion-network-mapping for lesion-deficit inference in patients with ischemic stroke. Our group investigates prediction of post-stroke deficits considering changes of structural brain network connectivity beyond traditional imaging markers. The extend of structural “dys-connection” is measured, either directly in DTI and fMRI data from affected patients or indirectly in reference connectomes from healthy subjects.
In collaboration with our research partners at the Institute of Computational Neuroscience at the UKE (head: Prof. C. Hilgetag), we apply an innovative inference approach based on game theory, the Multi-perturbation Shapley value Analysis (MSA), to better account for the dimensionalities inherent in brain anatomy and lesion patterns. MSA considers brain regions as ‘players’ in a game who interact to achieve a behavioral outcome and can also be used to quantify the interactions of the network elements (Zavaglia et al., 2015).
Collaboration: Institute of Computational Neuroscience, UKE
StroCare
Optimised Cross-sectoral, Coordinated Treatment of Stroke Patients With Patient-orientated Outcome Measurement
StroCare is an interventional study of Optimised Cross-sectoral, Coordinated Treatment of Stroke Patients With Patient-orientated Outcome Measurement. After stroke, patients often experience incisive changes in their health, daily routine and quality of life. The developed model of care (StroCare treatment) forms a cross-sectoral, structured and coordinated treatment pathway that integrates a patient-centred outcome evaluation. It aims to optimize the transition from acute inpatient treatment after an acute stroke to the ambulant neurological rehabilitation treatment.
StroCare is a multi-centred controlled interventional study with a pre-post design. The intervention comprises the development of an electronic portal solution for a safe and coordinated transmission of clinical data between the three participating hospitals and five stroke-specialized ambulant rehabilitation clinics, as well as a case-manager provided by the participating health insurance agency for patient support and aftercare coordination. Stroke patients in both groups are assessed after the index ischemic event and 12 months thereafter.
StroCare is a collaborative project between the University Medical Center Hamburg-Eppendorf (UKE), two hospitals with stroke units (Albertinenkrankenhaus Hamburg, Elbe-Kliniken Stade), five Neurological Rehabilitation Centers (RehaCentrum Hamburg, MEDICLIN Klinikum Soltau, Klinikum Bad Bramstedt, VAMED Klinik Geesthacht and VAMED Rehaklinik Damp), and the insurance company BARMER.
StroCare receives funding from the German Innovation Fund.
ClinicalTrials.gov Identifier, NCT04159324